Ovulation rate, prolificacy and pregnancy rate in goats treated with oral glycerol
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- Aguilar U, Hernández Cerón J, Domínguez Y, Gutiérrez C. Ovulation rate, prolificacy and pregnancy rate in goats treated with oral glycerol. Veterinaria México OA. 2016;3(1). doi: 10.21753/vmoa.3.1.360
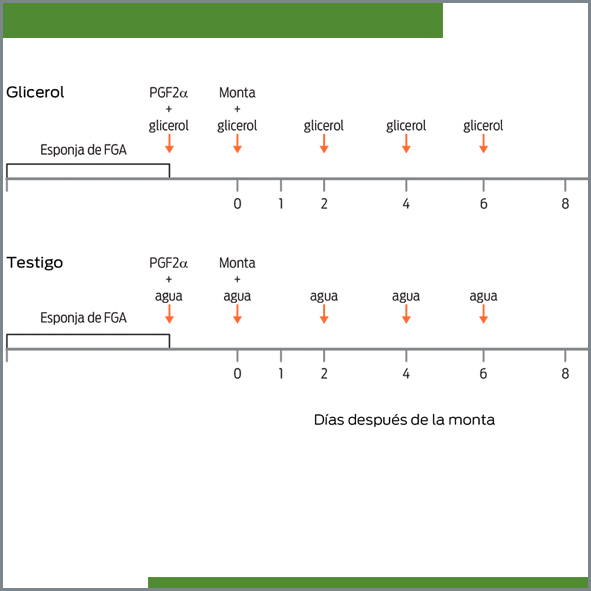
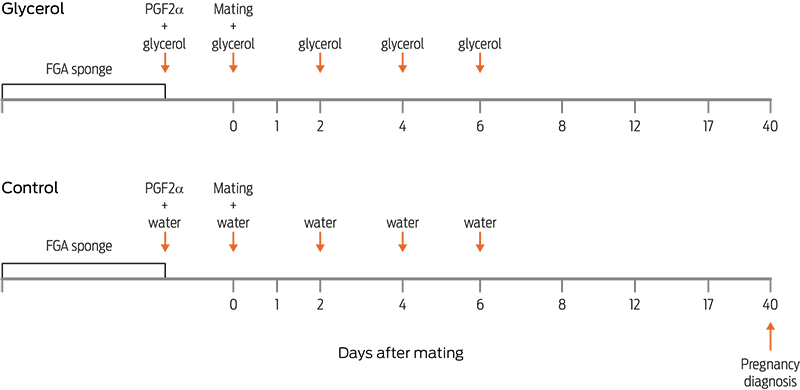
This study tested whether the oral administration of glycerol at the time of progestin removal and in the first 6 days of the estrous cycle increased the ovulation rate, prolificacy, and pregnancy rate in goats. Intravaginal sponges impregnated with fluorogestone acetate were inserted into 129 goats for 12 days; upon sponge removal, goats were randomly assigned to one of the two following treatment groups: the glycerol group (n = 65), which received an oral drench of 100 mL of glycerol upon sponge removal and was repeated on days 0, 2, 4, and 6 following estrus (estrus = day 0), and the control group (n = 64), which did not receive glycerol. The goats in estrus were mated, and their ovulation rate was determined by ultrasonography between days 8 and 12 of the estrous cycle. Pregnancy was diagnosed by ultrasonography on day 40, and the prolificacy was determined at birth. In 6 goats treated with glycerol and 5 controls, their insulin concentrations at 0, 2, 4, 8, and 12 h after the glycerol drench were determined by radioimmunoassay. The proportion of goats with multiple ovulations (glycerol = 71 vs control = 64) and the proportion of goats with multiple births (glycerol = 52 vs control = 56) were similar (P > 0.05) between treatments. Likewise, the pregnancy rate was similar (P > 0.05) between treatments (glycerol = 88 vs control = 85%). The insulin concentrations tended to be higher in goats treated with glycerol (P = 0.08). In conclusion, an oral drench of 100 mL of glycerol at the time of progestin withdrawal and in the first 6 days of the estrous cycle did not increase the ovulation rate, prolificacy, or pregnancy rate in goats.
Article Details
References
Arvizu R, Hernández Cerón J, Alberti A, Porras A, Valencia J. 1995. Inicio de la actividad ovárica posparto y características de la primera ovulación de cabras criollas paridas en dos épocas del año. Avances en Investigación Agropecuaria 4:9-15.
Augustin R, Pocar P, Wrenzycki C, Niemann H, Fischer B. 2003. Mitogenic and anti-apoptotic activity of insulin on bovine embryos produced in vitro. Reproduction 126:91-99.
Byrne AT, Southgate J, Brison DR, Leese HJ. 2002. Regulation of apoptosis in the bovine blastocyst by insulin and insulin-like growth factor (IGF) superfamily. Molecular Reproduction and Development 62:489-495.
Carrera-Chávez JM, Hernández-Cerón J, López-Carlos MA, Lozano-Domínguez RR, Molinar F, Echavarría-Cháirez FG, Bañuelos-Valenzuela R, Aréchiga CF. 2014. Superovulatory response and embryo development in ewes treated with two doses of bovine somatotropin. Animal Reproduction Science 151: 105-111.
Cooke RF, Cappellozza BI, Reis MM, Bohnert DW, Vasconcelos JL. 2012. Plasma progesterone concentration in beef heifers receiving exogenous glucose, insulin, or bovine somatotropin. Journal of Animal Science 90:3266–3273.
Downing JA, Joss J, Scaramuzzi RJ. 1995. Ovulation rate and the concentrations of gonadotrophins and metabolic hormones in ewes infused with glucose during the late luteal phase of the oestrous cycle. Journal of Endocrinology 146:403-410.
Dupont J, Scaramuzzi RJ, Reverchon M. 2014. The effect of nutrition and metabolic status on the development of follicles, oocytes and embryos in ruminants. Animal 8:1031–1044.
Fouladi-Nashta AA, Campbell KHS. 2006. Dissociation of oocyte nuclear and cytoplasmic maturation by the addition of insulin in cultured bovine antral follicles. Reproduction 131; 449-460.
García E. 1981. Modificaciones al Sistema de Clasificación Climática de Köppen. 5ª edición. México, D. F., México: Instituto de Geografía, UNAM.
Garret JE, Geisert RD, Zavy MT, Morgan GL. 1988. Evidence for maternal regulation of early conceptus growth and development in beef cattle. Journal of Reproduction and Fertility 84:437-446.
Gutiérrez CG, Oldham J, Bramley TA, Gong JG, Campbell BK, Webb R. 1997. The recruitment of ovarian follicles is enhanced by increased dietary intake in heifers. Journal of Animal Science 75:1876-1884.
Gutierrez CG, Ferraro S, Martinez V, Saharrea A, Cortez C, Lassala A, Basurto H, Hernandez Ceron J. 2011. Increasing ovulation quota: more than a matter of energy. Acta Scientiae Veterinariae 39 (Suppl 1):305-316.
Hernández-Cerón J, Gutiérrez CG. La somatotropina bovina recombinante y la reproducción en bovinos, ovinos y caprinos. Agrociencia 47: 35-45. 2013.
Muñoz-Gutiérrez M, Blache D, Martin GB, Scaramuzzi RJ. 2004. Ovarian follicular expression of mRNA encoding the type I IGF receptor and IGF-binding protein-2 in sheep following five days of nutritional supplementation with glucose, glucosamine or lupins. Reproduction 128:747-756.
Ortega LA, Hernández-Cerón J, Gutiérrez CG. 2010. La administración oral de glicerol después de la inseminación incrementa el porcentaje de concepción en vacas Holstein. Técnica Pecuaria en México 48:69-74
Rodríguez-Iglesias RM, Ciccioli NH, Irazoqui H, Giglioli C. 1996. Ovulation rate in ewes after single oral glucogenic dosage during a ram-induced follicular phase. Animal Reproduction Science 44:211-221.
Russel AJF, Doney JM, Gunn RG. 1969. Subjective assessment of body fat in live sheep. Journal of Agricultural Science 72:451-454.
Scaramuzzi RJ, Campbell BK, Downing JA, Kendall NR, Khalid M, Muñoz-Gutiérrez M, Somchit A. 2006. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reproduction Nutrition Development 46:339-354.
Silanikove N. 2000. The physiological basis of adaptation in goats to harsh environments. Small Ruminant Research 35:181-193.
Spicer, LJ, Echternkamp SE. 1995. The ovarian insulin andinsulin-like growth factor system with an emphasis on domestic animals. Domestic Animal Endocrinology 12:223–245.
Suguna K, Mehrotra S, Agarwal SK, Hoque M, Shanker U, Singh SK, Varshney VP. 2009. Effect of exogenous insulin administration on ovarian function, embryo/ fetal development during pregnancy in goats. Animal Reproduction Science 111:202-213.
Thatcher WW, Meyer MD, Danet-Desnoyers G. 1995. Maternal recognition of pregnancy. Journal of reproduction and Fertility 49 (Suppl.), 15–28.
Trabue S, Scoggin K, Tjandrakusuma S, Rasmussen MA, Reilly PJ. 2007. Ruminal Fermentation of Propylene Glycol and Glycerol Journal of Agricultural and Food Chemistry 55:7043–7051
Viñoles C, Forsberg M, Martin GB, Cajarville C, Repetto J, Meikle A. 2005. Shortterm nutritional supplementation of ewes in low body condition affects follicle development due to an increase in glucose and metabolic hormones. Reproduction 129:299-309.
Zabuli J, Tanaka T, Lu W, Kamomae H. 2010. Intermittent nutritional stimulus by short-term treatment of high-energy diet promotes ovarian performance together with increases in blood levels of glucose and insulin in cyclic goats. Animal Reproduction Science 122:288-293.
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.