Anesthetic evaluation of a novel phospholipid-free 1% propofol microemulsion formulation in dogs
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- Aquino I, Gutiérrez-Blanco E, Ocampo L, Gutiérrez L, Bernard-Bernard MJ, Sumano H. Anesthetic evaluation of a novel phospholipid-free 1% propofol microemulsion formulation in dogs. VetMéxOA. 2019;6(3). doi: 10.22201/fmvz.24486760e.2019.3.654.
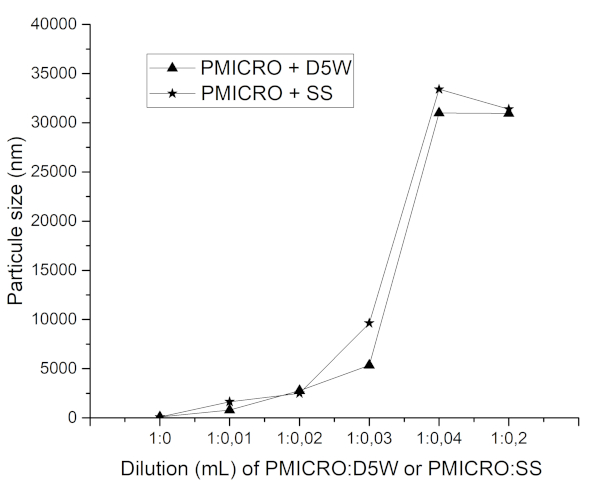
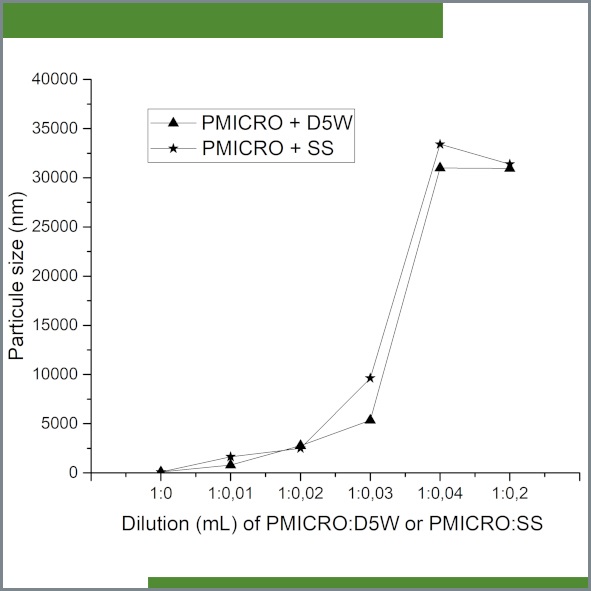
Figure 1. Particle size (nm) of a phospholipid-free 1% propofol microemulsion (PMICRO) diluted with either sterile 0.89% saline solution (SS) or 5% glucose solution (D5W) at different concentrations. Particle size was measured 5 min after dilution.
Article Details
References
Sumano H, Pérez N, Izquierdo P, Castellanos J. Anestesia general con propofol en perros mediante infusion continua. Experiencias clinicas. Vet México. 1994;25(3):199-205.
Muir W, Gadawski J. Cardiovascular effects of a high dose of romifidine in propofol-anesthetized cats. Am J Vet Res. 2002;63(9):1241-6. DOI: https://doi.org/10.2460/ajvr.2002.63.1241
Selmi AL, Figueiredo JP, Mendes GM, Lavor LMS, Machado PML. Infusão contínua de propofol em gatos pré-medicados com cetamina-midazolam. Arq Bras Med Veterinária e Zootec [Internet]. 2005;57(3):295-9 [cited 2016 Nov 27]. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-09352005000300003&lng=en&nrm=iso&tlng=pt DOI: https://doi.org/10.1590/S0102-09352005000300003
Sim JY, Lee SH, Park DY, Jung JA, Ki KH, Lee DH, et al. Pain on injection with microemulsion propofol. Br J Clin Pharmacol. 2009;67(3):316-25. DOI: https://doi.org/10.1111/j.1365-2125.2008.03358.x
Borwankar RP, Lobo LA, Wasan DT. Emulsion stability - kinetics of flocculation and coalescence. Colloids and Surfaces. 1992;69(2-3):135-46. DOI: https://doi.org/10.1016/0166-6622(92)80224-P
Adams H. Veterinary pharmacology and therapeutics. 9th ed. Iowa: Wiley-Blackwell; 2001.
Sumano H, Ocampo L. Manual de farmacología clínica para pequeñas especies. 4th ed. Mexico: Imagen Editorial Yire; 2009.
Ezquerra J, Vives M. Anestesia práctica de pequeños animales. 1st ed. Madrid: McGraw Hill-Interamericana; 1992. 200 p.
Marsico F, Cediel R, Gómez de Segura IA, Tendillo FJ, Criado AB. Un nuevo anestésico intravenoso: propofol : Clínica Vet pequeños Anim [Internet]. 1991;11(4):0231-9. Available from: http://ddd.uab.cat/record/69982
Tsagogiorgas C, Theisinger S, Heesch E, Krebs J, Holm R, Beck G, et al. Evaluation of pharmacokinetic properties and anaesthetic effects of propofol in a new perfluorohexyloctane (F6H8) emulsion in rats. A comparative study. Int J Pharm [Internet]. 2015;486(1-2):69-76. Available from: http://dx.doi.org/10.1016/j.ijpharm.2015.03.037 DOI: https://doi.org/10.1016/j.ijpharm.2015.03.037
Thibaut J, Rivera T, Ahumada F. Anestesia endovenosa en perros mediante el uso de propofol en dosis única, premedicado con acepromazina-atropina y xilazina-atropina. Arch Med Vet [Internet]. 2002;34(1):25-35 [cited 2016 Nov 24]. Available from: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0301-732X2002000100003&lng=en&nrm=iso&tlng=en DOI: https://doi.org/10.4067/S0301-732X2002000100003
Zorrilla-Vaca A, Escandón-Vargas K, Brand-Giraldo V, León T, Herrera M, Payán A. Bacterial contamination of propofol vials used in operating rooms of a third-level hospital. Am J Infect Control [Internet]. 2016;44(1):e1-3. Available from: http://dx.doi.org/10.1016/j.ajic.2015.08.009 DOI: https://doi.org/10.1016/j.ajic.2015.08.009
Cho J, Cho JC, Lee P, Lee M, Oh E. Formulation and evaluation of an alternative triglyceride-free propofol microemulsion. Arch Pharm Res. 2010;33(9):1375-87. DOI: https://doi.org/10.1007/s12272-010-0911-0
Dubey PK, Kumar A. Pain on injection of lipid-free propofol and propofol emulsion containing medium-chain triglyceride: A comparative study. Anesth Analg. 2005;101(4):1060-2. DOI: https://doi.org/10.1213/01.ane.0000166951.72702.05
Nakane M, Iwama H. A potential mechanism of propofol-induced pain on injection based on studies using nafamostat mesilate. Br J Anaesth. 1999;83(3):397-404. DOI: https://doi.org/10.1093/bja/83.3.397
Allchurch LG V, Crilly H. Fixed drug eruption to propofol. Anaesth Intensive Care. 2014;42(6):777-81. DOI: https://doi.org/10.1177/0310057X1404200614
Graham LF, Torres SMF, Jessen CR, Horne KL, Hendrix PK. Effects of propofol-induced sedation on intradermal test reactions in dogs with atopic dermatitis. Vet Dermatol. 2003;14(3):167-76. DOI: https://doi.org/10.1046/j.1365-3164.2003.00337.x
McHale SP, Konieczko K. Anaphylactoid reaction to propofol. Anaesthesia. 1992;47(10):864-5. DOI: https://doi.org/10.1111/j.1365-2044.1992.tb03150.x
Li X, Zhang Y, Fan Y, Zhou Y, Wang X, Fan C, et al. Preparation and evaluation of novel mixed micelles as nanocarriers for intravenous delivery of propofol. Nanoscale Res Lett [Internet]. 2011;6(1):275. Available from: http://www.nanoscalereslett.com/content/6/1/275 DOI: https://doi.org/10.1186/1556-276X-6-275
Wallentine CB, Shimode N, Egan TD, Pace NL. Propofol in a modified cyclodextrin formulation: First human study of dose-response with emphasis on injection pain. Anesth Analg. 2011;113(4):738-41. DOI: https://doi.org/10.1213/ANE.0b013e31822b8648
Lang BC, Yang J, Wang Y, Luo Y, Kang Y, Liu J, et al. An improved design of water-soluble propofol prodrugs characterized by rapid onset of action. Anesth Analg. 2014;118(4):745-54. DOI: https://doi.org/10.1213/ANE.0000000000000124
Minghella E, Benmansour P, Iff I, Senior JM, Mosing M. Pain after injection of a new formulation of propofol in six dogs. Vet Rec [Internet]. 2010;167(22):866-7. Available from: http://veterinaryrecord.bmj.com/cgi/doi/10.1136/vr.c5736 DOI: https://doi.org/10.1136/vr.c5736
SAGARPA. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. [Internet]. Diario oficial de la federación. México; 1999. p. 57. Available from: http://www.senasica.gob.mx/?doc=743
Bayne KAL. Environmental Enrichment of Nonhuman Primates, Dogs and Rabbits Used in Toxicology Studies. Toxicol Pathol. 2004;31(1 Suppl):132-7. DOI: https://doi.org/10.1080/01926230390175020
Sumano H, Ocampo L. Farmacología veterinaria. 4th ed. México: Aranda; 2016.
World Health Organization. Stability testing of active pharmaceutical ingredients and finished pharmaceutical products, Annex 10. WHO Expert Comm Specif Pharm Prep. 2018;(52):309-51.
Martinez-Taboada F, Leece EA. Comparison of propofol with ketofol, a propofol-ketamine admixture, for induction of anaesthesia in healthy dogs. Vet Anaesth Analg [Internet]. 2014;41(6):575-82. Available form: http://dx.doi.org/10.1111/vaa.12171 DOI: https://doi.org/10.1111/vaa.12171
Bigby SE, Beths T, Bauquier S, Carter JE. Postinduction apnoea in dogs premedicated with acepromazine or dexmedetomidine and anaesthetized with alfaxalone or propofol. Vet Anaesth Analg. 2017;44(5):1007-15. DOI: https://doi.org/10.1016/j.vaa.2016.10.004
Bjur KA, Cannon BC, Fine AL, Ritter MJ, Schueler KE, Nemergut ME. Propylene Glycol Toxicity in Adolescent with Refractory Myoclonic Status Epilepticus. Case Rep Pediatr [Internet]. 2017;2017:1-3. Available from: https://www.hindawi.com/journals/cripe/2017/2979486/ DOI: https://doi.org/10.1155/2017/2979486
Nanopolytox. Toxicological impact of nanomaterials derived from processing, weathering and recycling of polymer nanocomposites used in various industrial applications [Internet]. España; 2016. Available from: https://cordis.europa.eu/project/rcn/94382/brief/es
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.