Resistencia de nematodos gastrointestinales en borregos a la administración simple y combinada de antihelmínticos, benzimidazoles y closantel, en tres localidades de México
Contenido principal del artículo
Resumen
ISSN: 2448-6760
Cómo citar este artículo:
- Alcalá Canto Y, Sumano López HS, Ocampo Camberos L, Gutiérrez L,Tapia Pérez G. Resistencia de nematodos gastrointestinales en borregos a la administración simple y combinada de antihelmínticos, benzimidazoles y closantel, en tres localidades de México. Veterinaria México OA. 2016;3(4). doi: 10.21753/vmoa.3.4.374
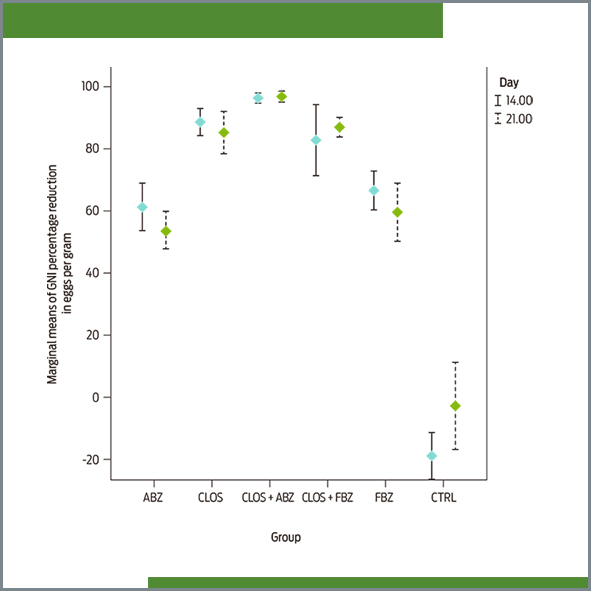
En la producción ovina se requiere de una constante evaluación de la carga parasitaria y la eficacia de tratamientos existentes para su adecuado manejo. En este estudio, se evaluó la administración de cinco tratamientos para eliminar nematodos gastrointestinales en borregos (reducción de huevos por gramo de heces, EPG) en tres granjas productoras de ovinos en México: Texcoco, Estado de México; Hueytanmalco, Puebla, y Tlaltizapán de Zapata, Morelos. A los borregos en estas granjas, rutinariamente se les administraron derivados de ivermectina y benzimidazol durante dos años consecutivos. Para determinar si estos desparasitantes con diferentes propiedades farmacológicas disminuyeron la excreción de huevos en heces (GIN), se administró closantel (CLOS), albendazol (ABZ) y fenmbendazol (FBZ) solos, y en combinaciones de CLOS + ABZ y CLOS + FBZ a cinco grupos de borregos, y se compararon con un grupo control sin tratamiento, en cada granja (n = 28 por rebaño). La resistencia antihelmíntica se determinó mediante la Prueba de Reducción en Conteo de Huevos Fecales (FECRT), tal como lo recomienda la “World Association for the Advancement of Veterinary Parasitology”. Se colectaron muestras fecales a los 14 y 21 días después del tratamiento. La resistencia antihelmíntica se determinó con base en la reducción de la media aritmética de la cuenta de huevos fecales con un 95 % de límite de confianza. De acuerdo con la FECRT, los nematodos desarrollaron resistencia a CLOS, ABZ, FBZ y CLOS+FBZ, debido a que el porcentaje medio de la reducción de EPG fue ≤ 95 % con un límite de confianza de ≤ 90 %. En contraste, la susceptibilidad de los nematodos a los antihelmintos se confirmó para la combinación CLO + ABZ ya que se redujo la expulsión de huevos fecales por 96.46 ±3.04 % (día 14), y 96.88 ±3.04 % (día 21). Con base en la identificación morfométrica de larvas, los géneros más abundantes fueron Haemonchus spp., Cooperia spp. y Teladorsagia spp. en todas las granjas antes del tratamiento. En conclusión, el uso de la combinación de los antihelmínticos closantel con albendazol puede reducir el desarrollo de la resistencia antihelmíntica en nematodos gastrointestinales.
* * * * * * * * * *
Equipo Editorial de Veterinaria México OA.Traducción del resumen original en inglés.Detalles del artículo
Citas
Besier B. New anthelmintics for livestock: the time is right. Trends Parasitol. 2007;23(1):21-4. doi: 10.1016/j.pt.2006.11.004.
Encalada-Mena L, Tuyub-Solis H, Ramirez-Vargas G, Mendoza-de-Gives P, Aguilar-Marcelino L, Lopez-Arellano ME. Phenotypic and genotypic characterisation of Haemonchus spp. and other gastrointestinal nematodes resistant to benzimidazole in infected calves from the tropical regions of Campeche State, Mexico. Vet Parasitol. 2014(1873-2550):246-54.
Chan-Perez JI, Torres-Acosta JF, Rodriguez-Vivas RI, Villegas-Perez SL. Reduction of benzimidazole resistance in established Haemonchus contortus populations in goats using a single infection with a benzimidazole-susceptible isolate. J Helminthol. 2015;89(1475-2697):641-5.
Höglund J, Gustafsson K, Ljungström BL, Engström A, Donnan A, Skuce P. Anthelmintic resistance in Swedish sheep flocks based on a comparison of the results from the faecal egg count reduction test and resistant allele frequencies of the beta-tubulin gene. Vet Parasitol. 2009;161(1-2):60-8. doi: 10.1016/j.vetpar.2008.12.001.
Falzon LC, O’Neill TJ, Menzies PI, Peregrine AS, Jones-Bitton A, vanLeeuwen J, et al. A systematic review and meta-analysis of factors associated with anthelmintic resistance in sheep. Prev Vet Med. 2014;117(2):388-402. doi: 10.1016/j.prevetmed.2014.07.003.
Zvinorova PI, Halimani TE, Muchadeyi FC, Matika O, Riggio V, Dzama K. Breeding for resistance to gastrointestinal nematodes - the potential in low-input/output small ruminant production systems. Vet Parasitol. 2016;225:19-28. doi: 10.1016/j.vetpar.2016.05.015.
Torres-Acosta JF, Mendoza-de-Gives P, Aguilar-Caballero AJ, Cuéllar-Ordaz JA. Anthelmintic resistance in sheep farms: update of the situation in the American continent. Vet Parasitol. 2012;189(1):89-96. doi: 10.1016/j.vetpar.2012.03.037.
Torres-Acosta JF, Molento M, Mendoza de Gives P. Research and implementation of novel approaches for the control of nematode parasites in Latin America and the Caribbean: is there sufficient incentive for a greater extension effort? Vet Parasitol. 2012;186(1-2):132-42. doi: 10.1016/j.vetpar.2011.11.053.
Hoste H, Torres-Acosta JFJ. Non chemical control of helminths in ruminants: Adapting solutions for changing worms in a changing world. Vet Parasitol. 2011;180:144-54.
Molento MB, Fortes FS, Pondelek DA, Borges FeA, Chagas AC, Torres-Acosta JF, et al. Challenges of nematode control in ruminants: focus on Latin America. Vet Parasitol. 2011;180(1-2):126-32. doi: 10.1016/j.vetpar.2011.05.033.
Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20(10):477-81. doi: 10.1016/j.pt.2004.08.001.
Kaplan RM, Vidyashankar AN. An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol. 2012;186(1-2):70-8. doi: 10.1016/j.vetpar.2011.11.048.
Kupˇcinskas T, Stadalien˙e I, ˇSark¯unas M, Riˇskeviˇcien˙e V, Várady M, Höglund J, et al. Prevalence of anthelmintic resistance on Lithuanian sheep farms assessed by in vitro methods. Acta Vet Scand. 2015;57:88. doi: 10.1186/s13028-015-0179-y.
Rialch A, Vatsya S, Kumar RR. Detection of benzimidazole resistance in gastrointestinal nematodes of sheep and goats of sub-Himalyan region of northern India using different tests. Vet Parasitol. 2013;198(3-4):312-8. doi: 10.1016/j.vetpar.2013.09.018.
Torres-Acosta JF, Dzul-Canche U, Aguilar-Caballero AJ, Rodríguez-Vivas RI. Prevalence of benzimidazole resistant nematodes in sheep flocks in Yucatan, Mexico. Vet Parasitol. 2003;114(1):33-42.
Encalada-Mena LA, Duarte-Ubaldo EI, Vargaz-Magana JJ, Garcia-Ramirez MJ, Medina-Hernandez RE. Prevalencia de parásitos gastroentéricos de canidos en la ciudad de Escarcega, Campeche, Mexico. Universidad y Ciencia. 2011(2):209.
Silvestre A, Humbert JF. Diversity of benzimidazole-resistance alleles in populations of small ruminant parasites. Int J Parasitol. 2002;32(7):921-8.
Silvestre A, Leignel V, Berrag B, Gasnier N, Humbert JF, Chartiere C, et al. Sheep and goat nematode resistance to anthelmintics: pro and cons among breeding management factors. Vet Res. 2002;33(5):465-80. doi: 10.1051/vetres:2002033.
Besier RB, Lyon J, Kieran PJ. The effect of moxidectin against benzimidazole- and levamisole-resistant nematodes of sheep in Western Australia. Aust Vet J. 1993;70(11):422-3.
Besier RB. Targeted treatment strategies for sustainable worm control in small ruminants. Trop Biomed. 2008;25(1 Suppl):9-17.
Nunes R, dos Santos L, Bastianetto E, Andrade de Oliveira D, Alves B, Brasil F. Frequency of benzimidazole resistance in Haemonchus contortus populations isolated from buffalo, goat and sheep herds. Rev Bras Parasitol Vet.. 2013;22(4):548-53.
Figueroa CJA, Jasso VC, Liébano HE, Martínez LP, Rodríguez VRI, Zárate RJJ. Examen Coproparasitoscópico. In: Técnicas para el diagnóstico de parásitos con importancia en salud pública y veterinaria. DF, México: AMPAVE-CONASA;2015. 517 p.
Coles GC, Jackson F, Pomroy WE, Prichard RK, von Samson-Himmelstjerna G, Silvestre A, et al. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136(3-4):167-85. doi: 10.1016/j.vetpar.2005.11.019.
Levecke B, Rinaldi L, Charlier J, Maurelli MP, Bosco A, Vercruysse J, et al. The bias, accuracy and precision of faecal egg count reduction test results in cattle using McMaster, Cornell-Wisconsin and FLOTAC egg counting methods. Vet Parasitol. 2012;188(1-2):194-9. doi: 10.1016/j.vetpar.2012.03.017.
Sweeny JP, Robertson ID, Ryan UM, Jacobson C, Woodgate RG. Comparison of molecular and McMaster microscopy techniques to confirm the presence of naturally acquired strongylid nematode infections in sheep. Mol Biochem Parasitol. 2011;180(1):62-7. doi: 10.1016/j.molbiopara.2011.07.007.
van Wyk JA, Cabaret J, Michael LM. Morphological identification of nematode larvae of small ruminants and cattle simplified. Vet Parasitol. 2004;119(4):277-306. doi: 10.1016/j.vetpar.2003.11.012.
CSIRO. Report of the working party for the Animal Health Committee of the Standing Committee on Agriculture;1989. Contract No. 28.
Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, et al. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44(1-2):35-44.
Lyndal-Murphy M, Swain AJ, Pepper PM. Methods to determine resistance to anthelmintics when continuing larval development occurs. Vet Parasitol. 2014;199(3-4):191-200. doi: 10.1016/j.vetpar.2013.11.002.
Dobson RJ, Hosking BC, Jacobson CL, Cotter JL, Besier RB, Stein PA, et al. Preserving new anthelmintics: a simple method for estimating faecal egg count reduction test (FECRT) confidence limits when efficacy and/or nematode aggregation is high. Vet Parasitol. 2012;186(1-2):79-92. doi: 10.1016/j.vetpar.2011.11.049.
Levecke B, Rinaldi L, Charlier J, Maurelli MP, Morgoglione ME, Vercruysse J, et al. Monitoring drug efficacy against gastrointestinal nematodes when faecal egg counts are low: do the analytic sensitivity and the formula matter? Parasitol Res. 2011;109(3):953-7. doi: 10.1007/s00436-011-2338-z.
McKenna PB. A comparison of faecal egg count reduction test procedures. N Z Vet J. 2006;54(4):202-3. doi: 10.1080/00480169.2006.36697.
McKenna PB. Further comparison of faecal egg count reduction test procedures: sensitivity and specificity. N Z Vet J. 2006;54(6):365-6. doi: 10.1080/00480169.2006.36726.
Bartram DJ, Leathwick DM, Taylor MA, Geurden T, Maeder SJ. The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet Parasitol. 2012;186(3-4):151-8. doi: 10.1016/j.vetpar.2011.11.030.
Leathwick DM, Ganesh S, Waghorn TS. Evidence for reversion towards anthelmintic susceptibility in Teladorsagia circumcincta in response to resistance management programmes. Int J Parasitol Drugs Drug Resist. 2015;5(1):9-15. doi: 10.1016/j.ijpddr.2015.01.001.
McKenna PB. The use of benzimidazole-levamisole mixtures for the control and prevention of anthelmintic resistance in sheep nematodes: an assessment of their likely effects. N Z Vet J. 1990;38(2):45-9. doi: 10.1080/00480169.1990.35614.
Leathwick DM, Miller CM, Sauermann CW, Candy PM, Ganesh S, Fraser K, et al. The efficacy and plasma profiles of abamectin plus levamisole combination anthelmintics administered as oral and pour-on formulations to cattle. Vet Parasitol. 2016;227:85-92. doi: 10.1016/j.vetpar.2016.07.031.
Leathwick DM. Managing anthelmintic resistance--parasite fitness, drug use strategy and the potential for reversion towards susceptibility. Vet Parasitol. 2013;198(1-2):145-53. doi: 10.1016/j.vetpar.2013.08.022.
Leathwick DM, Waghorn TS, Miller CM, Candy PM, Oliver AM. Managing anthelmintic resistance--use of a combination anthelmintic and leaving some lambs untreated to slow the development of resistance to ivermectin. Vet Parasitol. 2012;187(1-2):285-94. doi: 10.1016/j.vetpar.2011.12.021.
Learmount J, Taylor MA, Bartram DJ. A computer simulation study to evaluate resistance development with a derquantel-abamectin combination on UK sheep farms. Vet Parasitol. 2012;187(1-2):244-53. doi: 10.1016/j.vetpar.2011.12.033.
Schmahl G, Benini J. Treatment of fish parasites. 11. Effects of different benzimidazole derivatives (albendazole, mebendazole, fenbendazole) on Glugea anomala, Moniez, 1887 (Microsporidia): ultrastructural aspects and efficacy studies. Parasitol Res. 1998;84(1):41-9.
Swan GE. The pharmacology of halogenated salicylanilides and their anthelmintic use in animals. J S Afr Vet Assoc. 1999;70(2):61-70.
Gokbulut C, Bilgili A, Hanedan B, McKellar QA. Comparative plasma disposition of fenbendazole, oxfendazole and albendazole in dogs. Vet Parasitol. 2007;148(3-4):279-87. doi: 10.1016/j.vetpar.2007.06.028.
McKellar QA, Coop RL, Jackson F. The pharmacokinetics of albendazole metabolites following administration of albendazole, albendazole sulfoxide and netobimin to one-month- and eight-month-old sheep. Int J Parasitol. 1995;25(10):1207-12.
Torres-Acosta JFJ, Sandoval-Castro CA, Hoste H, Aguilar-Caballero AJ, Cámara-Sarmiento R, Alonso-Díaz MA. Nutritional manipulation of sheep and goats for the control of gastrointestinal nematodes under hot humid and subhumid tropical conditions. Small Rumin Res. 2012;103(1):28-40. doi: 10.1016/j.smallrumres.2011.10.016.
Torres-Acosta JF, Molento M, Fau-Mendoza de Gives P, Mendoza de Gives P. Research and implementation of novel approaches for the control of nematode parasites in Latin America and the Caribbean: is there sufficient incentive for a greater extension effort? Vet Parasitol. 2012(1873-2550):132-42.
License

Veterinaria México OA por Facultad de Medicina Veterinaria y Zootecnia de la Universidad Nacional Autónoma de México se distribuye bajo una Licencia Creative Commons Atribución 4.0 Internacional.
Basada en una obra en http://www.revistas.unam.mx
- Todos los artículos en Veterinaria México OA se publican bajo una licencia de Creative Commons Reconocimiento 4.0 Unported (CC-BY 4.0). Con esta licencia, los autores retienen el derecho de autor, pero permiten a cualquier usuario compartir, copiar, distribuir, transmitir, adaptar y hacer uso comercial de la obra sin necesidad de proporcionar un permiso adicional, siempre y cuando se otorgue el debido reconocimiento al autor o fuente original.
- Al utilizar esta licencia, los artículos en Veterinaria México OA cubren o exceden todos los requisitos fundacionales e institucionales para ser considerados de Acceso Abierto.
- Los autores no pueden utilizar material protegido por derechos de autor en su artículo a menos que ese material esté también disponible bajo una licencia igualmente generosa.